Abstract
Introduction: Amyloid light chain (AL) amyloidosis - a progressive disorder caused by misfolded light chains produced by plasma cells - is associated with high mortality, poor quality of life, and increased healthcare costs, particularly in newly diagnosed patients with advanced disease (Mayo 2012 Stage IV, median overall survival <6 months). Birtamimab is a monoclonal antibody designed to neutralize circulating soluble and deplete deposited insoluble amyloid by promoting phagocytic clearance. In 2018, the Phase 3 VITAL study - a multicenter, global, randomized, double-blind, placebo-controlled study (NCT02312206) conducted in newly diagnosed, treatment-naive patients with AL amyloidosis and cardiac involvement - was terminated based on a futility analysis of the primary endpoint (time to all-cause mortality [ACM] or time to cardiac hospitalization ≥91 days after first study drug infusion); the final hazard ratio (HR) numerically favored birtamimab + standard of care (SOC) over placebo + SOC (0.826 [95% CI: 0.574, 1.189]). Post hoc analysis of ACM over 9 months revealed a significant survival benefit (HR=0.413 [95% CI: 0.191, 0.895]) in patients at high risk for early death (i.e., Mayo 2012 Stage IV). Here we report the results of sensitivity analyses of ACM in the subgroup of patients with Mayo 2012 Stage IV AL amyloidosis, adjusting for key baseline variables.
Methods: For ACM, the hazard ratio and 90% two-sided confidence interval were estimated from the semi-parametric Cox Regression model stratified by randomization strata (i.e., Mayo Stage I/II vs III/IV, renal stage I vs II/III, and 6MWT [6-minute walk test] distance). Separately, baseline variables including age, sex, race, ethnicity, age at diagnosis, duration since diagnosis, NT-proBNP (N-terminal pro-brain natriuretic peptide), dFLC (difference between involved and uninvolved free light chain), FLC (free light chain), NYHA (New York Heart Association) class, troponin-T, and 6MWT distance were added to the Cox Regression model to evaluate the impact on the overall survival benefit. All adjudicated deaths prior to 9 months were included in the analysis. Patients who had no events were censored at 9 months.
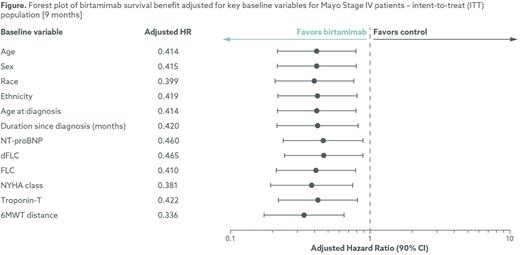
Results: Of the 260 patients enrolled in the VITAL study, 77 (29.6%) were characterized as Mayo 2012 Stage IV at baseline, 38 randomized to birtamimab + SOC and 39 to placebo + SOC. These patients had a median age of 64 years and were primarily white (93.5%) and male (68.8%). Baseline demographic and clinical characteristics were generally balanced between the two treatment groups among these Mayo Stage IV patients. After adjustment for key baseline demographic, clinical, and laboratory variables, the adjusted hazard ratios with each of the baseline variables added separately to the Cox Regression model range from 0.336 to 0.465, with all the upper bounds of the 90% confidence interval less than 1 (Figure).
Conclusions: Birtamimab is the only investigational therapeutic that has shown a significant survival benefit in Mayo Stage IV AL amyloidosis patients. The survival benefit of birtamimab was consistent across all key baseline variables, including demographic factors (age, sex, race, ethnicity), clinical characteristics (age at diagnosis, duration since diagnosis, NYHA class, 6MWT distance), and laboratory parameters (NT-proBNP, dFLC, FLC, troponin-T), reinforcing the strength of the survival data in Mayo Stage IV patients. The AFFIRM-AL study, designed to confirm the VITAL study results in Mayo Stage IV AL amyloidosis patients, is currently active and enrolling.
Disclosures
Gertz:Ionis/Akcea: Other: personal fees; Prothena: Other: personal fees; Sanofi: Other: personal fees; Janssen: Other: personal fees; Aptitude Healthgrants: Other: personal fees; Ashfield: Other: personal fees; Juno: Other: personal fees; Physicians Education Resource: Other: personal fees; Abbvie: Membership on an entity's Board of Directors or advisory committees, Other: personal fees from Data Safety Monitoring Board; Johnson & Johnson: Other: personal fees; Celgene: Other: personal fees; Research to Practice: Other: personal fees; Sorrento: Other: personal fees; i3Health: Other: Development of educational materials for i3Health. Jin:Prothena Biosciences Inc.: Current Employment; Prothena Corporation Plc.: Current equity holder in private company. Conrad:Prothena Biosciences Inc.: Current Employment; Prothena Corporation Plc.: Current equity holder in private company. Nie:Prothena Biosciences Inc.: Current Employment; Prothena Corporation Plc.: Current equity holder in private company.
OffLabel Disclosure:
The Phase 3 VITAL study reports results for an investigational agent, birtamimab, for potential use in patients with Stage IV AL Amyloidosis.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal